To resolve this dilemma we make use of two important developments of the last decades: microscopic simulation and deterministic chaos. Specifically, we do in-class live simulations of model systems, and we invoke intuitive geometrical arguments to explore Gibbs' phase space.
PCs have recently become powerful enough to do a nontrivial simulation within 1-2 minutes, which is the attention span available in a lecture setting. Thus we are now in position to use the PC together with a data projector in the same way as any other setup for a lecture-hall experiment. In addition, using the Java technology we can make the simulation program available to students for exercises and projects.
Chaos theory has yielded a result which is of utmost practical importance in didactics: chaos can be found in systems with no more than two degrees of freedom. This means that we can actually d r a w the phase space and the energy surface (line, rather) of such a system and demonstrate that the microstates are indeed equally distributed. The`assumption' of equal a priori probability on the energy surface has thus become an experimental, demonstrable fact.
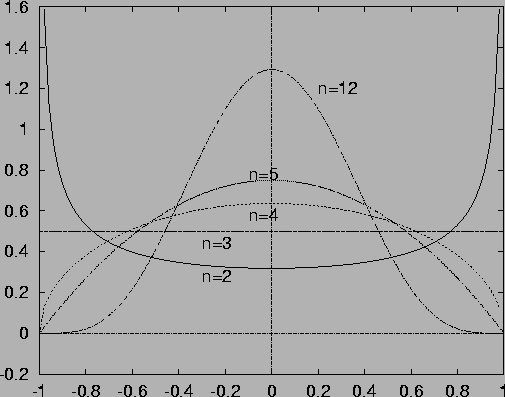
On the basis of this evidence we may now go on to more degrees of freedom. Studying hard discs systems with increasing numbers of particles we may invite students to explore the simple but surprising properties of multidimensional space. Since hard bodies have a hyperspherical energy surface, we have to deal only with very simple objects in phase space. In particular, we may predict and verify the distribution of one velocity component in a system of 1, 2, or more hard discs and hard balls. Very soon this distribution approaches a Gaussian (see Figure) and thus yields the Maxwell-Boltzmann distribution for the modulus of the particle velocity.
Having understood phase space and energy surfaces, we can go on to make the all-important transition from statistics to thermodynamics. The vehicle for this traversal is S, the entropy. We make the tentative assumption that S is given by the log of the phase space volume of the system. Next we demonstrate that this quantity has indeed the constitutive properties of the entropy: additivity and the fact that S governs the energy transfer between two systems in contact: dE/dS (temperature) must be equal in both systems. While this argument is rather too abstract for second-year students, it can be visualised easily by letting a given amount of energy be divided up between two systems in all possible proportions. It becomes evident that the most probable situation, having the largest phase space volume, is just the one given by the equal temperature law.
After these discoveries we are in a position to assemble all the basic facts of Statistical Physics commonly encountered in textbooks: ensembles, partition functions, free energies, quantum statistics, basic kinetic theory. A short writeup of the present paper, including four sample Java programs, may be accessed via http://www.ap.univie.ac.at/users/ves/grc2000/bx.